Safety and antiviral activity of triple combination broadly neutralizing monoclonal antibody therapy against HIV-1: a phase 1 clinical trial
Abstract
HIV-1 therapy with single or dual broadly neutralizing antibodies (bNAbs) has shown viral escape, indicating that at least a triple bNAb therapy may be needed for robust suppression of viremia.
Analysis of the HVTN 702 Phase 2b-3 HIV-1 vaccine trial in South Africa assessing RV144 antibody and T-cell correlates of HIV-1 acquisition risk
Abstract
Background
The ALVAC/gp120 + MF59 vaccines in the HVTN 702 efficacy trial did not prevent HIV-1 acquisition. Vaccine-matched immunological endpoints that were correlates of HIV-1 acquisition risk in RV144 were measured in HVTN 702 and evaluated as correlates of HIV-1 acquisition.
Methods
Defining variant-resistant epitopes targeted by SARS-CoV-2 antibodies: A global consortium study
Antibody-based therapeutics and vaccines are essential to combat COVID-19 morbidity and mortality after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Multiple mutations in SARS-CoV-2 that could impair antibody defenses propagated in human-to-human transmission and spillover or spillback events between humans and animals.
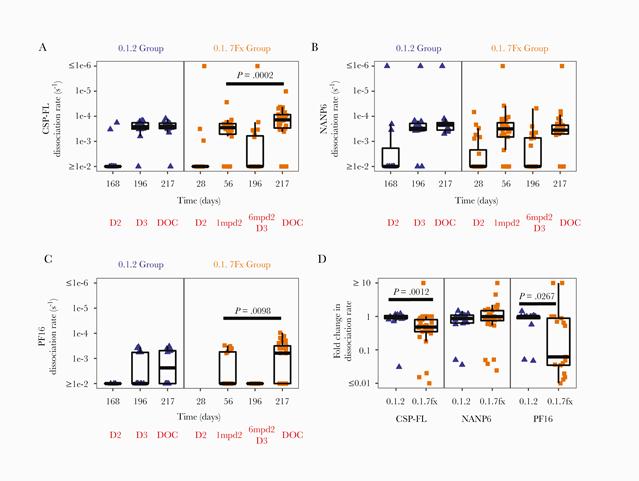
Subclass and avidity of circumsporozoite protein specific antibodies associate with protection status against malaria infection
RTS,S/AS01 is an advanced pre-erythrocytic malaria vaccine candidate with demonstrated vaccine efficacy up to 86.7% in controlled human malaria infection (CHMI) studies; however, reproducible immune correlates of protection (CoP) are elusive. To identify candidates of humoral correlates of vaccine mediated protection, we measured antibody magnitude, subclass, and avidity for Plasmodium falciparum (Pf) circumsporozoite protein (CSP) by multiplex assays in two CHMI studies with varying RTS,S/AS01B vaccine dose and timing regimens.
Validation of a Triplex Pharmacokinetic Assay for Simultaneous Quantitation of HIV-1 Broadly Neutralizing Antibodies PGT121, PGDM1400, and VRC07-523-LS
The outcome of the recent Antibody Mediated Prevention (AMP) trials that tested infusion of the broadly neutralizing antibody (bnAb) VRC01 provides proof of concept for blocking infection from sensitive HIV-1 strains. These results also open up the possibility that triple combinations of bnAbs such as PGT121, PGDM1400, as well as long-lasting LS variants such as VRC07-523 LS, have immunoprophylactic potential. PGT121 and PGDM1400 target the HIV-1 V3 and V2 glycan regions of the gp120 envelope protein, respectively, while VRC07-523LS targets the HIV-1 CD4 binding site.
Meta-analysis of HIV-1 vaccine elicited mucosal antibodies in humans
We studied mucosal immune responses in six HIV-1 vaccine trials investigating different envelope (Env)-containing immunogens. Regimens were classified into four categories: DNA/vector, DNA/vector plus protein, protein alone, and vector alone. We measured HIV-1-specific IgG and IgA in secretions from cervical (n = 111) and rectal swabs (n = 154), saliva (n = 141), and seminal plasma (n = 124) and compared to corresponding blood levels. Protein-containing regimens had up to 100% response rates and the highest Env-specific IgG response rates.